Procurement – application capability
The product development group of this organization had always preferred outsourcing of trial management capabilities, including data management, to clinical research organizations. The decision was to select and license an integrated clinical trial management suite directly by the organization.
This was since for each trial they had a range of vendors, systems, data standards and internal teams managing them. Given that the number of trials was planned to duplicate within a year, they were looking for economies of scale, with better licensing terms and process standardization, harmonization and also to enable reuse of the data for exploratory use cases as per FAIR principles.
Other desired outcomes were to:
- improve performance, particularly for the principal investigators
- higher readiness for regulatory inspections
- planned data security audits
- increase robustness (both process and system related)
(Case Study)

Solutions proposed
The client considered the following options
- developing an in-house solution
- work with a Clinical Research Organization to sublicense a single system that they would support
- procuring a new system and licensing it directly
Given that none of the vendor options would support the full end to end process and functional requirements, a number of variations were also considered.
Client contribution
Availability of both practitioners and senior management for timely decisions in the selection process.
Process maps.
Scoring of functional aspects by user experts, business consultants and regulatory experts.
Input into architecture, security and privacy assessment.
Sign off of requirements and scoring and final decision.
Outline
After a target capability model was agreed, a structured approach was followed.
The Clinical Development unit and others identified vendor candidates and procurement engaged in RFI.
Requirements, both functional and non functional were agreed.
RFP issued for software suites, assessment of responses, bid defence, shortlist, define pilot(s).
New RFP issued for B2B identity management services.
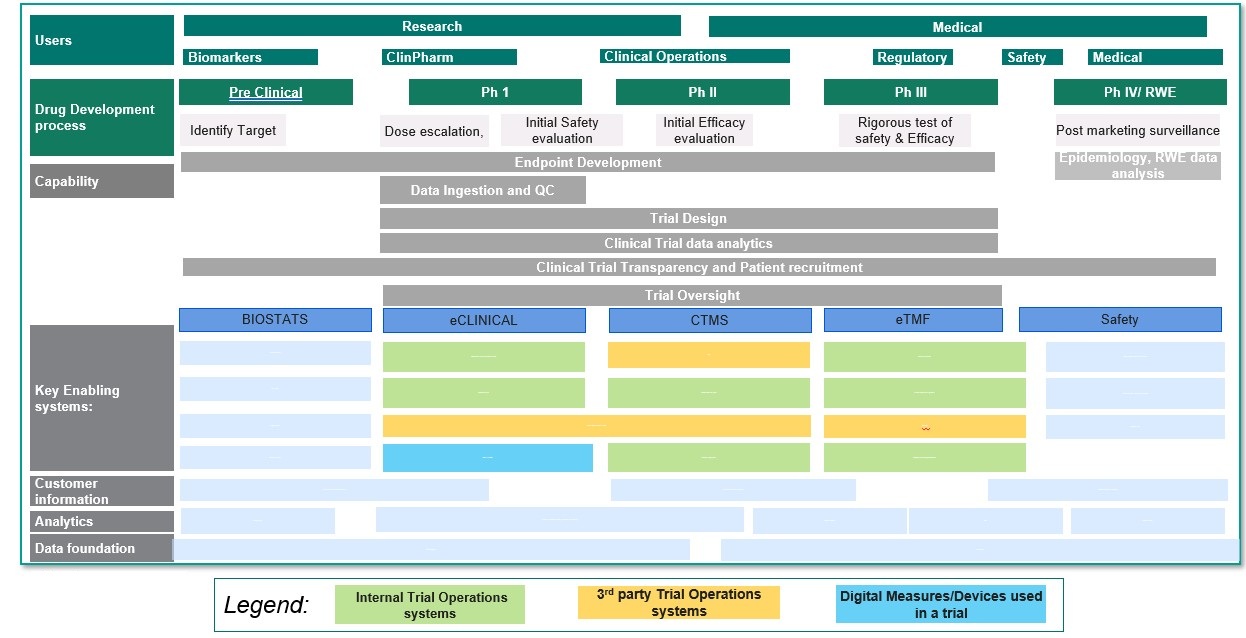
Requirements
The team involved product development, global procurement, business services procurement, external consultants, product manager and various IT functions.
Preliminaries
The client SMEs confirmed the goals and strategy.
The procurement approach and future governance of the initiative with drug supply units and other stakeholders was agreed.
Sign off of baseline criteria and scoring.
Deliverables and outcomes
Our deliverables included: non functional requirements and criteria (architecture and operations, security, data privacy, etc), evaluation of responses including scoring, evaluation demos, report and recommendation on included/excluded, input to the audit of shortlisted vendors. Future data flows. Advice regarding operationalization approach: internal vs external teams.
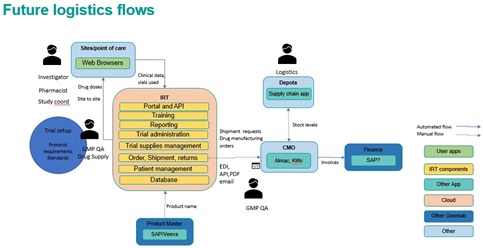
Next steps
Once two systems were shortlisted, the solution architectures were outlined to agree short pilots. These supported negotiation of production licenses for system and business consulting services (from each vendor).
Learnings
One key challenge was to ensure that non functional requirements were considered, issuing them directly to the vendors would have helped. There was also reluctance to deselect vendors at the pace IT had anticipated. Also, given operating these systems was not common in the client and there was no precedent for a role for the IT department (despite it being of value to), there was a risk the business department would hire technical services from the vendor, reducing opportunities for economies of scale from the IT function. Finally, limited visibility of the trial pipeline and hiring plans meant that assessing the level of local and remote support that each product would need from the vendor became very iterative. This also limited the extent to which common work packages but that were required for all trials could be built once (for example, platform level libraries and technical integrations could have be reused in multiple trials, however this was only leveraged to a limited extent).
Due to the selected vendor not delivering promised new features as agreed, the client had to engage and to ultimately select their second choice vendor. We helped to enable that switch.
Feedback
The client was very pleased with our input and commented on the impartiality, the ability to anticipate operational challenges.
The business stakeholders continued to seek our input during the operationalization of the system that they selected and they recommended us for other projects.
Notes: Key factors for fast engagement include appropriate detail of planning including availability of key resources, prior identification of relevant accelerators and our flexibility to adapt to your ways of working.
Our use case descriptions may combine outputs and benefits from discrete engagements, even if typically in the same client.

